Leishmaniasis Fact Sheet
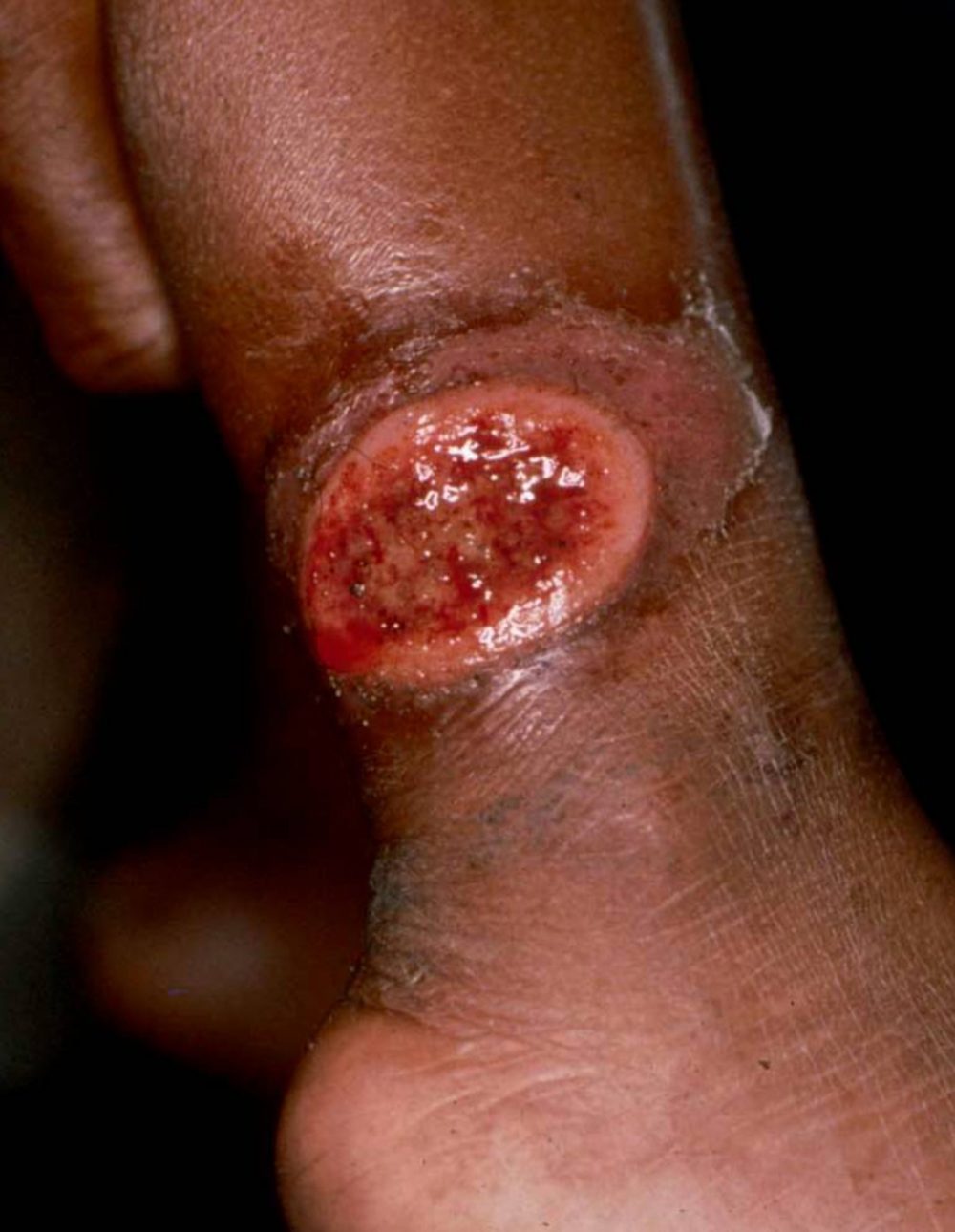
Leishmaniasis is caused by a protozoa parasite from over 20 Leishmania species and is transmitted to humans by the bite of infected female phlebotomine sandflies. Over 90 sandfly species are known to transmit Leishmania parasites
Fact Sheet
Key facts
- There are 3 main forms of leishmaniases – visceral (also known as kala-azar and the most serious form of the disease), cutaneous (the most common), and mucocutaneous.
- Leishmaniasis is caused by the protozoan Leishmania parasites which are transmitted by the bite of infected female phlebotomine sandflies.
- The disease affects some of the poorest people on earth, and is associated with malnutrition, population displacement, poor housing, a weak immune system and lack of financial resources.
- Leishmaniasis is linked to environmental changes such as deforestation, building of dams, irrigation schemes, and urbanization.
- An estimated 700 000–1 million new cases and 20 000 to 30 000 deaths occur annually.
- Only a small fraction of those infected by Leishmania parasites will eventually develop the disease.
Leishmaniasis is caused by a protozoa parasite from over 20 Leishmania species and is transmitted to humans by the bite of infected female phlebotomine sandflies. Over 90 sandfly species are known to transmit Leishmania parasites. There are 3 main forms of the disease:
- Visceral leishmaniasis (VL), also known as kala-azar is fatal if left untreated in over 95% of cases. It is characterized by irregular bouts of fever, weight loss, enlargement of the spleen and liver, and anaemia. It is highly endemic in the Indian subcontinent and in East Africa. An estimated 50 000 to 90 000 new cases of VL occur worldwide each year. In 2015, more than 90% of new cases reported to WHO occurred in 7 countries: Brazil, Ethiopia, India, Kenya, Somalia, South Sudan and Sudan. The kala-azar elimination programmes in South-East Asia are making sustained progress towards elimination, and cases are declining in the three major endemic countries: Bangladesh, India and Nepal.
- Cutaneous leishmaniasis (CL) is the most common form of leishmaniasis and causes skin lesions, mainly ulcers, on exposed parts of the body, leaving life-long scars and serious disability. About 95% of CL cases occur in the Americas, the Mediterranean basin, the Middle East and Central Asia. Over two thirds of new CL cases occur in 6 countries: Afghanistan, Algeria, Brazil, Colombia, Iran (Islamic Republic of) and the Syrian Arab Republic. An estimated 0.6 million to 1 million new cases occur worldwide annually.
- Mucocutaneous leishmaniasis leads to partial or total destruction of mucous membranes of the nose, mouth and throat. Over 90% of mucocutaneous leishmaniasis cases occur in Bolivia (the Plurinational State of), Brazil, Ethiopia and Peru.
Transmission
Leishmania parasites are transmitted through the bites of infected female phlebotomine sandflies. The epidemiology of leishmaniasis depends on the characteristics of the parasite species, the local ecological characteristics of the transmission sites, current and past exposure of the human population to the parasite, and human behaviour. Some 70 animal species, including humans, have been found as natural reservoir hosts of Leishmania parasites.
East Africa
In East Africa, there are frequent outbreaks of visceral leishmaniasis in the northern acacia–balanite savanna and the southern savanna and forest areas where sandflies live around termite mounds. Humans are considered the main reservoir of the Leishmania parasites causing visceral leishmaniasis in this part of Africa. Cutaneous leishmaniasis occurs in the highlands of Ethiopia and other places in East Africa, where increased human–fly contact occurs in villages built on rock hills or river banks, which are the natural habitat of hyraxes.
North Africa-Eurasia
In North Africa-Eurasia, cutaneous leishmaniasis is the main form of the disease. Agricultural projects and irrigation schemes can increase the prevalence of cutaneous leishmaniasis as people who have no immunity to the disease move in to work on the projects. Large outbreaks in densely populated cities also occur, especially during war and large-scale population migration. The parasites causing cutaneous leishmaniasis live mainly on humans or rodents.
Leishmania-HIV co-infection
Leishmania-HIV coinfected people have high chance of developing the full-blown clinical disease, and high relapse and mortality rates. Antiretroviral treatment reduces the development of the disease, delays relapses and increases the survival of the coinfected patients. High Leishmania-HIV coinfection rates are reported from Brazil, Ethiopia and the state of Bihar in India.
Major risk factors
Socioeconomic conditions
Poverty increases the risk for leishmaniasis. Poor housing and domestic sanitary conditions (such as a lack of waste management or open sewerage) may increase sandfly breeding and resting sites, as well as their access to humans. Sandflies are attracted to crowded housing as these provide a good source of blood-meals. Human behaviour, such as sleeping outside or on the ground, may increase risk. The use of insecticide-treated bednets reduces risk.
Malnutrition
Diets lacking protein-energy, iron, vitamin A and zinc increase the risk that an infection will progress to kala-azar.
Population mobility
Epidemics of both cutaneous and visceral leishmaniasis are often associated with migration and the movement of non-immune people into areas with existing transmission cycles. Occupational exposure as well as widespread deforestation remain important factors. For example, people settling in areas that used to be forests may be moving near sandflies’ habitat. This can lead to a rapid increase in cases.
Environmental changes
Environmental changes that can affect the incidence of leishmaniasis include urbanization, domestication of the transmission cycle and the incursion of agricultural farms and settlements into forested areas.
Climate change
Leishmaniasis is climate-sensitive, and strongly affected by changes in rainfall, temperature and humidity. Global warming and land degradation together affect the epidemiology of leishmaniasis in a number of ways:
- changes in temperature, rainfall and humidity can have strong effects on vectors and reservoir hosts by altering their distribution and influencing their survival and population sizes;
- small fluctuations in temperature can have a profound effect on the developmental cycle of Leishmania promastigotes in sandflies, allowing transmission of the parasite in areas not previously endemic for the disease;
- drought, famine and flood resulting from climate change can lead to massive displacement and migration of people to areas with transmission of Leishmania, and poor nutrition could compromise their immunity.
Diagnosis and treatment
In visceral leishmaniasis, diagnosis is made by combining clinical signs with parasitological, or serological tests (such as rapid diagnostic tests). In cutaneous and mucocutaneous leishmaniasis serological tests have limited value. In cutaneous leishmaniasis, clinical manifestation with parasitological tests confirms the diagnosis.
The treatment of leishmaniasis depends on several factors including type of disease, concomitant pathologies, parasite species and geographic location. Leishmaniasis is a treatable and curable disease, which requires an immunocompetent system because medicines will not get rid of the parasite from the body, thus the risk of relapse if immunosuppression occurs. All patients diagnosed as with visceral leishmaniasis require prompt and complete treatment. Detailed information on treatment of the various forms of the disease by geographic location is available in the WHO technical report series 949, “Control of leishmaniasis“.
Prevention and control
Prevention and control of leishmaniasis requires a combination of intervention strategies because transmission occurs in a complex biological system involving the human host, parasite, sandfly vector and in some causes an animal reservoir host. Key strategies for prevention are listed below:
- Early diagnosis and effective case management reduces the prevalence of the disease and prevents disabilities and death. Early detection and prompt treatment of cases help to reduce transmission and to monitor the spread and burden of disease. Currently there are highly effective and safe anti-leishmanial medicines particularly for visceral leishmaniasis. Access to these medicines has significantly improved thanks to a AHO-negotiated price scheme and a medicine donation programme through AHO.
- Vector control helps to reduce or interrupt transmission of disease by controlling sandflies, especially in domestic conditions. Control methods include insecticide spray, use of insecticide–treated nets, environmental management and personal protection.
- Effective disease surveillance is important. Prompt data reporting is key to monitor and take action during epidemics and situations with high case fatality rates under treatment.
- Control of animal reservoir hosts is complex and should be tailored to the local situation.
- Social mobilization and strengthening partnerships – mobilization and education of the community with effective behavioural change interventions must always use locally tailored communication strategies. Partnership and collaboration with various stakeholders and other vector-borne disease control programmes is critical.
AHO Action Plan
AHO’s work on leishmaniasis control involves:
- supporting national leishmaniasis control programmes to produce updated guidelines and make disease control plans;
- raising awareness and advocacy on the global burden of leishmaniasis, and promoting equitable access to health services for disease prevention and case management;
- developing evidence-based policy guidelines, strategies and standards for leishmaniasis prevention and control, and monitoring their implementation;
- providing technical support to Member States to build sustainable, effective surveillance system and epidemic preparedness and response systems;
- strengthening collaboration and coordination among partners, stakeholders and other bodies;
- monitoring the global leishmaniasis situation, trends, progress in the disease control, and financing;
- providing diagnostic tests and antileishmanial medicines as last resort source;
- promoting research on effective leishmaniasis control including in the areas of safe, effective and affordable medicines, as well as diagnostic tools and vaccines; and
- facilitating the dissemination of research findings.
Budget for AHO Action Plan on Leishmaniasis
US$75 million
Please donate and let us eliminate Leishmaniasis
Sources: WHO, PAHO, NHS

